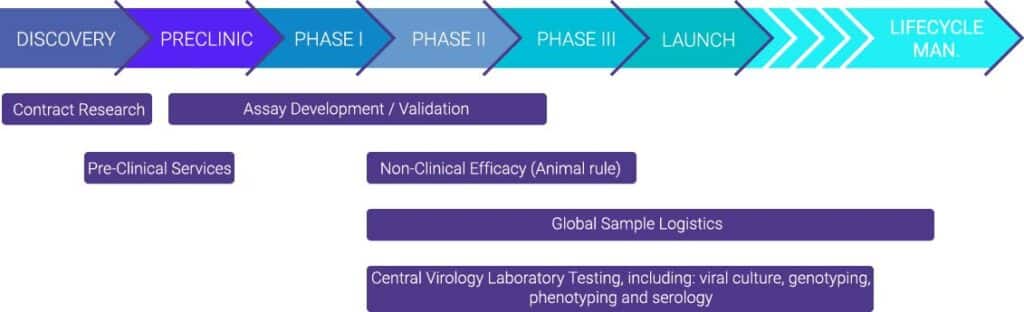
With our services, we assist the pharmaceutical, biotech and academic communities from early preclinical research, including in vivo models, through large-scale, late-stage clinical trial programs for vaccines, monoclonal antibodies, and antivirals. In our BSL3 containment laboratories, we offer fully validated assays, targeting highly pathogenic viruses.We offer a wide range of preclinical models, virological, molecular, and immunological assays. In addition, we offer a full suite of global logistics services.
With our inhouse R&D services we are expertise in developing and validating new assays, improving existing assays and implementing customer assays. Highly customized solutions are available including novel assay platforms such as The VirospotTM (trademark).
The assay platform has been developed for the detection and phenotypic characterization of influenza viruses in phase 2 and phase 3 clinical trials of new antiviral compounds. It combines classic virus culture techniques with automated sensitive detection of immunostained virus infected cells and is now also available in a format for antigenic characterization of influenza A(H3N2) viruses.
Our Services

Virological Assays
- We provide phenotyping/ viral culture
- We have a number of high-throughput plaque assays available, including high-throughput plaque reduction assays with a 96-well format
- Our viral assays include virus titration techniques, virus neutralization evaluation, virus inhibition analysis, ELISA and ELISpot/Fluoro spot assays, etc

Molecular Assays
- We conduct viral-loaded qPCRs
- We can provide real-time and multiplex PCR
- We offer next-generation sequencing methods for whole genomes and targeted genes, such as RNA-sequencing, B-Cell Receptor / T-Cell Receptor Sequencing, and Whole genome sequencing

Preclinical models
- We use the wild-type virus in our BSL2 and BSL3 facilities to provide confirmed animal models for studying a variety of viral targets such as Influenza, SARS-CoV-2, MERS, RSV, HSV and others.
- We provide in vitro assays as well as in vivo studies with a variety of animal models.
- We offer proof of concept studies for vaccines and anti viral components (PK studies, immunogenicity and efficacy testing).
Our experienced preclinical team offers you tailor-made advice for your project.

Global Logistics Services
- Preparation of virology sampling kits
- On-site sampling handling instruction and courier transport
- Sample tracking and tracing (e.g., guaranteeing temperature-controlled supply chain and online-sample timelines)
- Management of sample processing labs.
- Network of 41 processing labs

Virology Research Services
- Central and reference virology laboratory for phase I-IV clinical trials
- Research & Development services
- Vaccine and antiviral therapies
- Global surveillance programs

Reach out to our experts and see how we can help advance your clinical trial. You can also find out more about our vaccine lab services.
Contact Us
